The classification of minerals is primarily based on their chemical composition, which allows scientists and geologists to systematically organize them into distinct groups. Minerals may be classified based on their chemical composition. They are grouped and described under 17 chemical divisions, such as native elements, sulphides, oxides, carbonates, silicates, phosphates, sulphates, borates, and nitrates, as given by Dana and Ford (1948).
Of these, silicates are the predominant minerals in rocks of the Earth’s crust. Carbonates, oxides, sulphides, and phosphates are the other commonly found minerals.
Main Mineral Classes by Chemical Composition:
The following list provides the classification of all the common minerals according to their abundance:
- Silicate class
- Carbonate class
- Sulphate, phosphate, chromate, tungstate, molybdate, and vanadate classes
- Halide class
- Oxide and hydroxide classes
- Sulphide class
- Native element class
Silicate class:
The (SiO₄) tetrahedron forms the fundamental structural unit in all silicates, with a central silicon atom (Si) surrounded by four oxygen atoms (O) in a tetrahedral arrangement. The bond between silicon and oxygen remains very strong. Silicates form the largest group of minerals found in rocks.
Nearly 95 per cent of the rocks contain silicon and oxygen in addition to aluminium, magnesium, iron, and calcium. Fig. 1 shows the important silicate minerals: quartz, feldspars, pyroxenes, amphiboles, micas, olivines, and garnets.
The feldspar group alone (orthoclase, plagioclase [albite], oligoclase, labradorite, bytonite, and anorthite) constitutes about 60 per cent of the crust, and quartz nearly 10 per cent. The silica minerals predominantly occur in igneous rocks and metamorphic rocks, but also appear in sedimentary rocks.
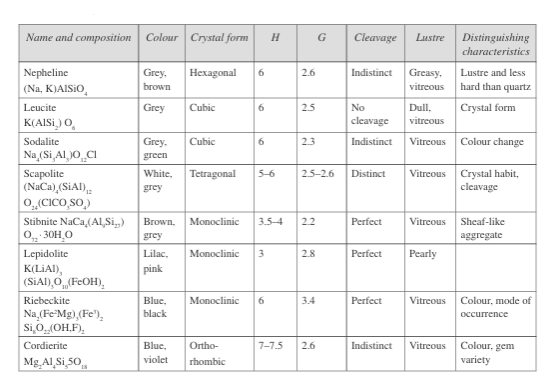
Fig.1: Properties of silicate minerals
Feldspar, pyroxene, amphibole, olivine, garnet, zircon, sillimanite, kyanite, staurolite, topaz, epidote, muscovite, biotite, and tourmaline talc comprise the silicates, apart from the silica mineral quartz and opal (amorphous) varieties.
Fig. 1 presents the properties of the other minerals. Clay minerals belong to a subclass of silicates called phyllosilicates, which are very fine-grained and possess swelling properties. One can identify them by a study under an electron microscope and also by the X-ray diffraction method and differential thermal analysis. Fig. 2 lists the properties of these clay minerals, which are of importance in engineering construction.
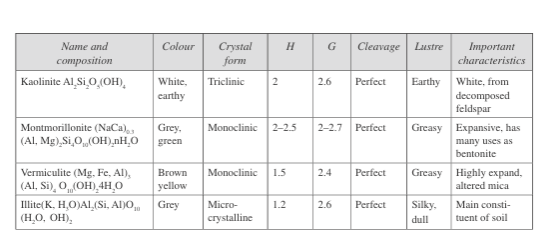
Fig. 2: Properties of clay minerals under phyllosilicates
Carbonate class:
The carbonate (CO₃) class forms isolated structures and does not form chains, rings, or layers like silicates. The important minerals of this group include calcite and aragonite (calcium carbonate), dolomite (calcium/magnesium carbonate), and siderite (iron carbonate).
Other carbonate minerals include magnesite (magnesium carbonate), rhodochrosite (manganese carbonate), smithsonite (zinc carbonate), witherite (barium carbonate), strontianite (strontium carbonate), and cerussite (lead carbonate). One can find rocks containing carbonate minerals such as limestone and dolomite in marine environments where shell and planktonic life abound.
Carbonates also occur in evaporitic settings and in karst regions, where the dissolution and reprecipitation of carbonates form big cavities, including stalactites and stalagmites. Carbonate minerals also appear in metamorphic rocks as marble (calcium carbonate).
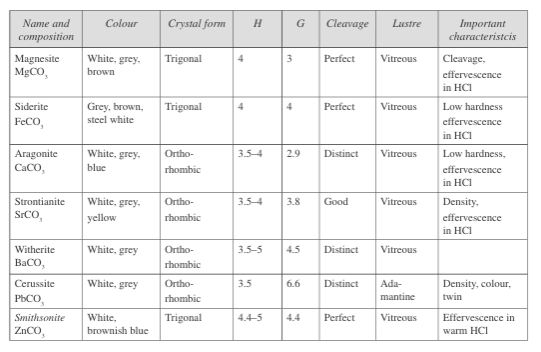
Fig.3: Properties of carbonate class minerals
The nitrates and borate minerals also contain carbonates, which usually form through evaporation from salt lakes. Fig.3 gives a description of the common carbonate minerals.
Sulphate class:
Sulphate occurs in sulphate minerals as SO₄²⁻. The (SO₄) tetrahedron forms a complex anion or group in which four O atoms surround a central S atom at the corners of the tetrahedron.
These tetrahedra remain isolated in the structure and do not form groups, chains, or layers as in silicates. Sulphate minerals usually form in evaporitic settings where slow evaporation of saline water leads to the formation of both sulphates and halides at the water-sediment interface.
They also occur in hydrothermal vein systems as gangue minerals along with sulphide ore minerals. In addition, sulphates result from secondary oxidation of original sulphide minerals.
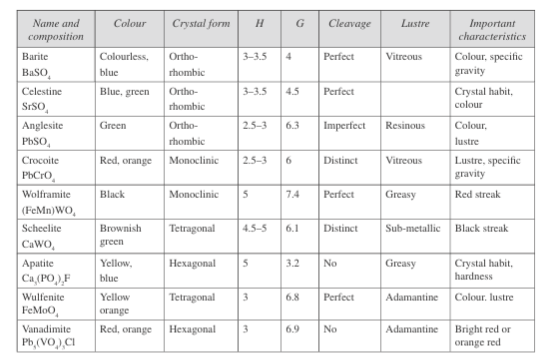
Fig. 4: Properties of sulphate class minerals
The common sulphate minerals include anhydrite (calcium sulphate), celestine (strontium sulphate), barium sulphate, and gypsum (hydrated calcium sulphate). The sulphate class also contains phosphate, chromate, molybdate, vanadate, and tungstate minerals. Fig. 4 presents the minerals of this class.
Halide class:
The halide group of minerals includes simple compounds such as the natural salts fluorite (calcium fluoride), halite (sodium chloride), and sylvite (potassium chloride), in which the halogen element bonds to an alkali metal.
Halides, like sulphates, frequently form in evaporative environments such as salt lakes and landlocked seas like the Dead Sea and the Great Salt Lake in the US. The halide group also includes the fluoride, chloride, bromide, and iodide minerals.
Chlorides form mostly through evaporation from solutions, while fluorides typically occur in igneous rocks and their associated pegmatite and hydrothermal veins. Fig.5 outlines the halide class minerals.

Fig.5: Properties of halide class minerals
Oxide and hydroxide class:
Many mineable ores belong to the oxide class, which contains metallic minerals made up of oxygen and one or more metals. These minerals typically have chemical structures of closely packed large oxygen atoms with the small metal atom.
They also preserve the best record of changes in the Earth’s magnetic field. These minerals generally occur as precipitates near the Earth’s surface and as oxidation products of other minerals in the near-surface weathering zone. They also appear as accessory minerals in igneous rocks and metamorphic rocks of the Earth’s crust and mantle.
Common oxide minerals include hematite, magnetite (iron oxide), chromite (iron chromium oxide), rutile (titanium dioxide), spinel (magnesium aluminium oxide), zincite (zinc oxide), pyrolusite (manganese oxide), uraninite (uranium oxide), cassiterite (tin oxide), and ice (hydrogen oxide).
Because of their high resistance to weathering and transport, they also concentrate in sediment beds. In hydroxides, the oxygen is completely or partially replaced by the OH group. Hydroxides tend to be less hard and less dense than oxides.
These minerals typically form in the upper weathering zone of ore deposits, produced by alteration of the primary minerals. Some common hydroxide minerals include gibbsite, Al(OH)₃; manganite, MnO(OH); diaspore, AlO(OH); and goethite, FeO(OH).
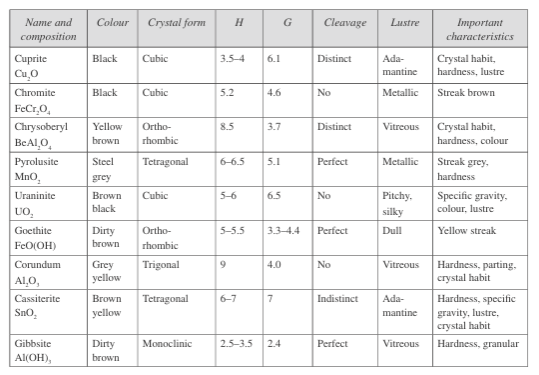
Fig.6: Properties of oxide and hydroxide class minerals
Fig.6 outlines the properties of important minerals of this class. Some of the common oxide minerals in this class such as magnetite, hematite, ilmenite, and spinel appear in Section Fig. 2 under ore-forming minerals and precious stones.
Sulphide class:
The sulphides contain a large number of minerals, many of which hold economic importance as metallic ores. A sulphide consists of an oxygen-free compound of sulphur and one or more metals.
Most sulphides exhibit metallic character with strong colour, streak, and metallic lustre. These minerals are also mostly opaque and possess high densities. Unlike pure metal, they are usually brittle.
The common sulphides include pyrite (iron sulphide), copper sulphide, chalcopyrite (copper iron sulphide), nickeline (nickel sulphide), sphalerite (zinc sulphide), galena (lead pyrite), and cinnabar (mercury sulphide). Fig. 7 describes the properties of minerals in the sulphide class.
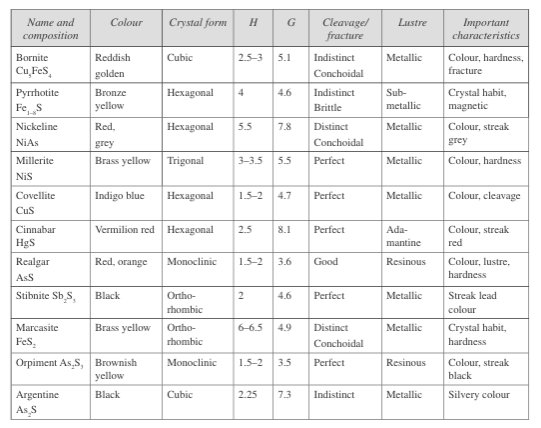
Fig. 7: Properties of sulphide class minerals
Native element class:
The chemical elements occurring in the Earth’s crust are few, and none exists in large quantities. Some minerals like gold, silver, and diamond are well known for their valuable properties.
The element class includes native metals and intermetallic elements (gold, silver, copper, mercury, platinum, and iron), semimetals, and non-metals (arsenic, antimony, bismuth, graphite, sulphur, and tellurium).
These metals consist of spherical cations such as Au⁺ in close packings, mostly in cubic closest-packed structures. This group also includes natural alloys like electrum (a natural alloy of gold and silver) and phosphide.
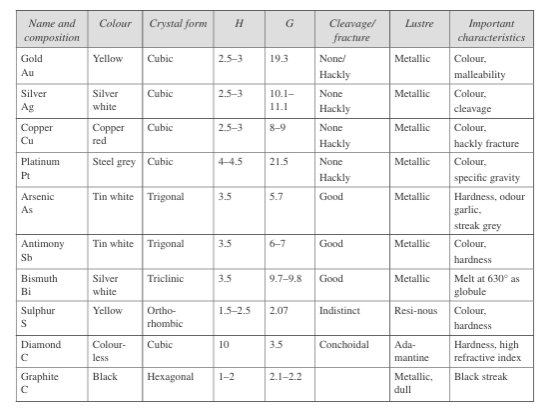
Fig. 8: Properties of minerals of native element class