Clay minerals consist of crystalline materials that give soil its plasticity and cohesion. A clayey soil, along with non-clay minerals like quartz, feldspar, mica, and calcite, often includes one or two clay minerals made of microscopically thin sheets.
Chemically, clay minerals contain aluminum silicate or a mix of iron and magnesium silicate, though some also include alkaline earth elements. Structurally, they feature two primary building blocks: one with a tetrahedral configuration and the other with an octahedral configuration.
The tetrahedral unit holds a silica atom bonded with four hydroxyls, while the octahedral unit surrounds an aluminum, iron, or magnesium atom with six hydroxyls. Together, these units form densely packed sheets or layers.
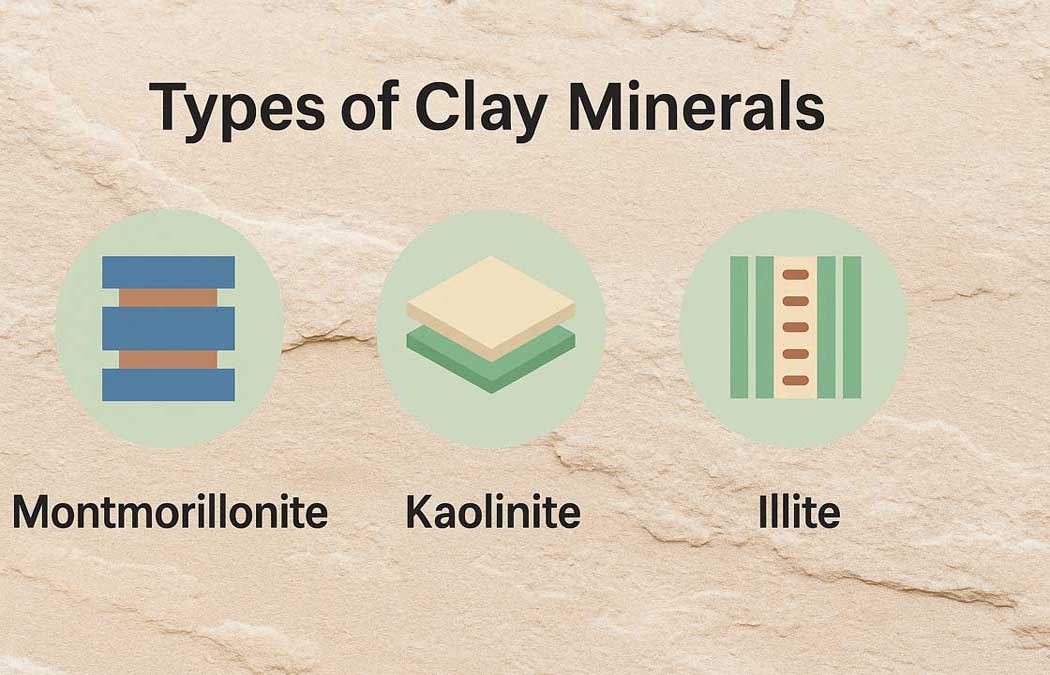
Types of Clay Minerals:
Soil commonly contains three main groups of clay minerals:
• Montmorillonite
• Kaolinite
• Illite
Montmorillonite:
Montmorillonite, an expansive clay mineral, has a sheet-like structure. Its formation involves an octahedral gibbsite sheet sandwiched between two silica sheets. Water bonds within these sheets cause the clay to swell.
Each thin sheet or platelet attracts a layer of adsorbed water nearly 20 times thicker than the sheet itself. Soils rich in montmorillonite show significant swelling and shrinkage.
In water-abundant conditions, this clay may split into individual layers. Bentonite—a type of montmorillonite derived from volcanic ash—serves as drilling mud. Its expansive nature prevents borehole wall collapse, though it can pose serious risks if present in building foundations.
Kaolinite:
Kaolinite, a non-swelling clay mineral, originates from kaoline, an altered form of feldspar—a key mineral in rocks like granite and pegmatite. Its structure contains gibbsite and silicate sheets.
Hydrogen bonds hold the layers firmly together, preventing water from entering the structure. As a result, kaolinite mixed with water shows minimal swelling.
Its fine platelets carry negative electromagnetic charges that attract thick layers of adsorbed water. This feature gives it excellent plasticity under wet conditions. The pottery industry widely uses kaolinite, often referred to as china clay.
Illite:
Illite is a non-expansive clay mineral. Although its structure resembles montmorillonite, illite replaces some silicates in the tetrahedral layers with aluminum. Potassium ions fill the gaps and hold the sheets together.
Unlike kaolinite, illite crystals in soil break easily into platelets that consist of a gibbsite layer between two silicate layers.