Thermodynamics focuses on the interactions between heat, work, and temperature, exploring how these elements connect to energy, entropy, and the physical characteristics of matter and radiation. These interactions are defined by three fundamental laws of thermodynamics, which provide a quantitative framework through measurable macroscopic physical quantities.
However, statistical mechanics helps to explain these phenomena at the microscopic level. The principles of thermodynamics are applicable across a vast range of fields in both science and engineering.
Thermodynamics:
The study of the relationship between heat and other forms of energy involved in a chemical or physical process.
Thermodynamics Laws:
The laws of thermodynamics are described below,
- Zeroth Law
- First Law: Describes the conservation of energy.
- Second Law: Establishes entropy and spontaneous processes.
- Third Law: Explains the impossibility of achieving absolute zero.
Zeroth Law:
The Zeroth law of thermodynamics states that when two systems are in thermodynamic equilibrium with a third system, the original two systems are in thermal equilibrium. If system A is in thermal equilibrium with system C and system B is in thermal equilibrium with system C, then systems A and B are in thermal equilibrium.
First Law:
It states that energy can be converted from one form to another but can’t be created or destroyed.
i.e ∆E= Q- W
change in energy = heat – work
So, let’s explore the three primary forms of heat transfer,
The methods by which heat can be transferred are convection, conduction, and radiation. In convection, thermal energy is circulated by a fluid sitting in a gravity field. Conduction involves the transfer of thermal energy through solids. Finally, in radiation, thermal energy is transferred via electromagnetic waves.
Second Law:
The entropy of the universe increases in spontaneous processes and remains unchanged in equilibrium processes.
∆S univ = ∆S (sys) + ∆S (surr) ≥O
- If the reaction produces more gas molecules, it is consumed ∆S is positive.
- If the total number of gas molecules diminishes, ∆S is negative
- If there is no net change in the total no of gas molecules, then ∆S may be positive/ negative.
Third Law:
The entropy of a perfect crystalline substance is zero at the absolute zero of temperature.
If the crystal is impure or has defects, its entropy is more significant than zero, even at O K.
Entropy, represented by ‘S,’ is a measure of disorder or randomness within a closed system. This directly correlates to the number of microstates a system has access to, and essentially, the more microstates available, the higher the entropy of the system. The microstate where the system’s energy is at its minimum is called its ground state.
At absolute zero, or zero Kelvin, specific phenomena occur within a closed system:
– The system lacks any heat.
– All atoms and molecules reside at their lowest energy levels.
Thus, at absolute zero, a system can have only one microstate, the ground state. The third law of thermodynamics states that the entropy of a system at absolute zero is precisely zero.
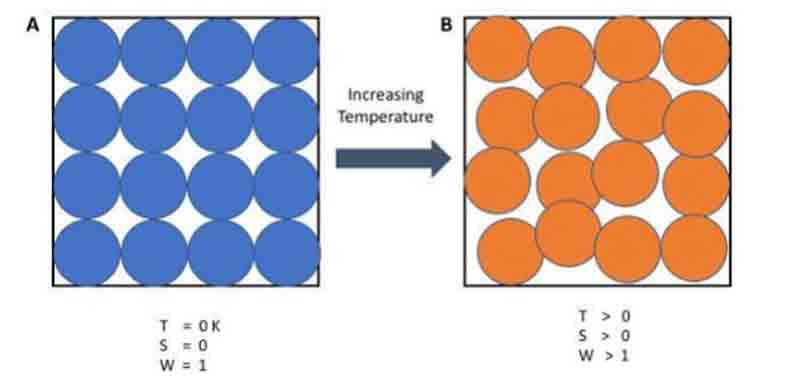
This law was developed between 1906 and 1912 by German chemist Walter Nernst.