Metamorphic Reaction:
The study of metamorphic reactions provides vital information about the pressure and temperature conditions of metamorphism. During metamorphism, chemical reactions between components take place to produce a new mineral assemblage. The agents of change are Temperature, Pressure, and Fluids.
Types of Metamorphic Reactions:
The metamorphic reaction is of four types:
- Solid-solid phase transformation
- Solid-solid net-transfer
- Dehydration/Hydration
- Decarbonation/Carbonation
Progressive metamorphic sequences usually involve the gradual dehydration or decarbonation of a rock mass. Hydration is a characteristic feature of retrogressive metamorphism.
Solid-solid phase transformation:
Some metamorphic reactions involve only solid phases. It does not involve a change in composition, only a rearrangement of the crystal structure. Minerals with the same composition but different crystal structures are termed as polymorphs.
There are three minerals with the composition of Al2SiO5. Each has unique crystal structures and is stable under definite pressure-temperature conditions.
Such differing forms with identical composition are called polymorphs. If pyrophyllite is dehydrated under high-temperature conditions, the polymorph of Al2SiO5 formed would be the mineral kyanite (the densest polymorph). With continued heating, the original andalusite or kyanite will invert to sillimanite, the highest temperature Al2SiO5 polymorph:
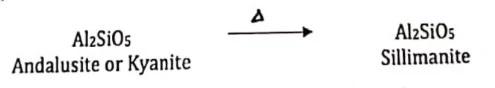
Polymorphic Reaction: A mineral reacts to for a polymorph of that mineral.
Solid-solid net-transfer:
Solid-solid net transfer involves only solids, too, but the difference from polymorphic transformation is that solid-solid net transfer involves solids of different compositions, and thus, minerals must diffuse from one site to another for the reaction to proceed.
Example:
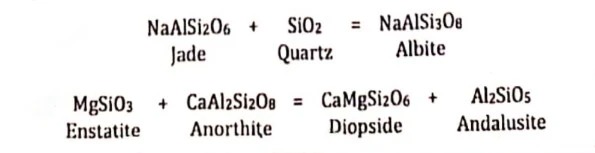
If minerals contain volatiles, the volatiles must be conserved in the reaction so that no fluid phase is generated or consumed. For example:

This reaction involves hydrous phases, but conserves H2O. It may therefore be treated as a solid-solid net transfer reaction.
Dehydration/Hydration:
Dehydration metamorphic reaction involves the expulsion or incorporation of water (H2O). When the rock is buried to a depth at which temperatures of about 300°C obtained, a chemical reaction sets in, and the kaolinite and quartz are transformed into pyrophyllite and water.
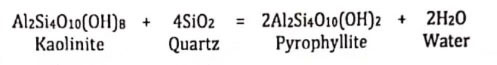
If heating and burial continue, another dehydration sets in at about 400°C, in which the pyrophyllite is transformed into andalusite, quartz, and water.

Dehydration reactions are common in the prograde metamorphism of rocks that initially contain water, e.g., shales. After the water has escaped, the rock becomes virtually anhydrous.
Retrogressive (Retrograde) metamorphism often involves the addition of water, the reaction being referred to as hydration, i.e.

Becarbonation/Carbonation Reactions:
Decarbonation occurs during the metamorphism of carbonate rocks such as limestone. This reaction involves the evolution or consumption of CO2.
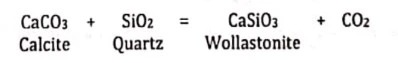
The formation of the CaWO4 (Calcium Tungstate) scheelite when tungstate in the form of WO3 moves from granite into a limestone contact. The reaction can be expressed as:
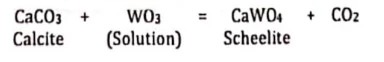
Reactions involving gas phases are also known as volatilization or devolatilization reactions. These reactions can also occur with other gases, such as CH4 (methane), H2, H2S, O2, and NH4 (ammonia) – but they are not as common.
- Dehydration reactions are reactions that liberate H20.
- Decarbonation reactions are reactions that liberate CO2.