Photochemical smog is produced when ultraviolet light from the sun reacts with nitrogen oxides in the atmosphere. It is visible as a brown haze and is most prominent during the morning and afternoon, especially in densely populated, warm cities. Cities that experience this smog daily include Los Angeles, Sydney, Mexico City, Beijing, and many more.
What is Photochemical Smog?
Photochemical smog is a mixture of pollutants formed when nitrogen oxides and volatile organic compounds (VOCs) react to sunlight, creating a brown haze above cities. It tends to occur more often in summer because that is when there is the most sunlight.
Formation:
Sunlight reacts with hydrocarbons and nitrogen oxides to form it. Photochemical smog typically contains ozone, nitric oxide, acrolein, formaldehyde, and peroxyacetyl nitrate (PAN) as its components. The formation process can be summarized as follows:
Burning fossil fuels emits hydrocarbons and nitrogen dioxide into the atmosphere. Sunlight interacts with these pollutants when they reach high concentrations in the air, leading to the following reactions:
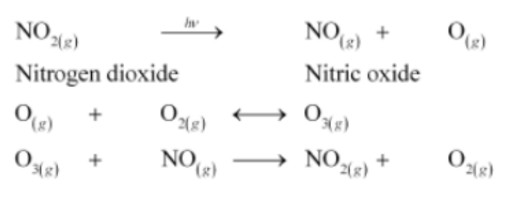
While ozone is toxic in nature, both NO2 and O3 are oxidizing agents. They react with the unburnt hydrocarbons in the air to produce formaldehyde, PAN, and acrolein.
3CH4 + 2O3 → 3CH2=O(formaldehyde) + 3H2O
Causes:
Sunlight, hydrocarbons, and nitrogen oxides cause photochemical smog. Driving cars, producing energy in fossil fuel power plants, or partially burning fossil fuels creates these pollutants.
Effects:
The presence of NO2 and O3 causes the corrosion of metals, stones, rubber, and painted surfaces, making the environment oxidizing. PAN, acrolein, and formaldehyde are other major components of photochemical smog.
PAN and ozone irritate the eyes, while nitric oxide (formed from NO2) irritates the nose and throat. At higher concentrations, it causes chest pain, headaches, throat dryness, and various respiratory ailments.
Measures to Control:
Burning fossil fuels and automobile fuels emits NO2 and hydrocarbons, which then form ozone, PAN, and other chemicals, resulting in photochemical smog.
To reduce the release of NO2 and hydrocarbons into the atmosphere, automakers recommend using catalytic converters. Planting species like Pinus, Juniparur, Quercus, Pyrus, and Vitis is also encouraged because they metabolize NO2 effectively.
Difference between Photochemical Smog and Classical Smog:
| Photochemical Smog | Classical Smog |
| It occurs in a dry, sunny climate. | It occurs in a cool, humid climate. |
| Its components are PAN, acrolein, ozone, formaldehyde, and nitric oxide. | Its components are smoke, fog, and Sulphur dioxide. |
| Its constituents are acrolein, ozone, formaldehyde, and nitric oxide. | Its constituents are smoke, fog, and sulphur dioxide. |
| It is oxidizing in nature. | It is reducing in nature. |
Conclusion
Photochemical smog is more than just an environmental issue; it’s a public health crisis. Understanding its causes and effects is the first step toward finding solutions. By taking action at both the individual and societal levels, we can ensure healthier, more sustainable cities for generations to come.