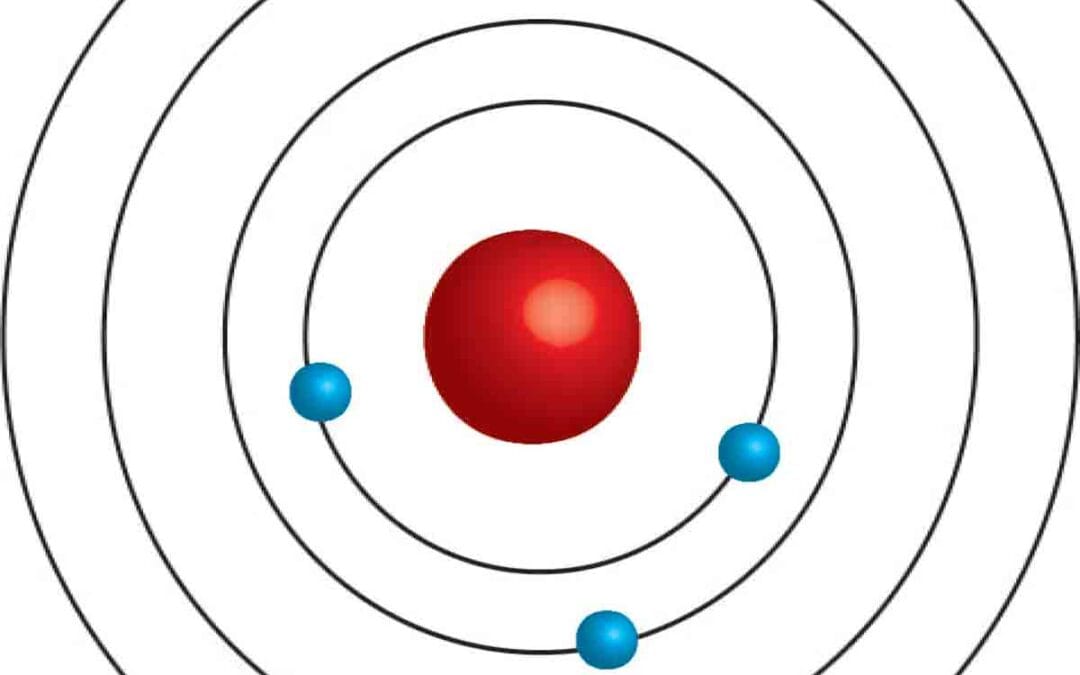
Niels Bohr introduced Bohr’s model of the atom as an enhancement of Rutherford’s atomic model. Rutherford had established a nuclear model that described the atom with a positively charged nucleus surrounded by negatively charged electrons. Bohr expanded on this concept by detailing that electrons orbit the nucleus in fixed paths, or shells, rather than moving freely between them. He also clarified that these orbits correspond to a specific energy level.
While Rutherford laid the groundwork by outlining the nuclear structure, Bohr provided a more comprehensive understanding by focusing on the behavior of electrons and their energy states.
Historical Background:
The Bohr’s model of atomic structure, crafted by Danish physicist and Nobel Prize winner Niels Bohr (1885–1962), marked a significant advancement in 1913. This model enhanced the earlier classical atomic theories proposed by J. J. Thomson and Ernest Rutherford by integrating the principles of quantum theory. While pursuing his doctoral dissertation at Copenhagen University, Bohr delved into Max Planck’s theories on quantum radiation. Following his graduation, Bohr collaborated with Thomson in England and later teamed up with Rutherford, during which he formulated his influential atomic structure model.
What is the Bohr Model?
Bohr’s model describes an atom featuring a tiny, positively charged nucleus encircled by negatively charged electrons. In this model, electrons farther from the nucleus possess more energy, while those nearer have less energy.
Structure of the Bohr’s Model:
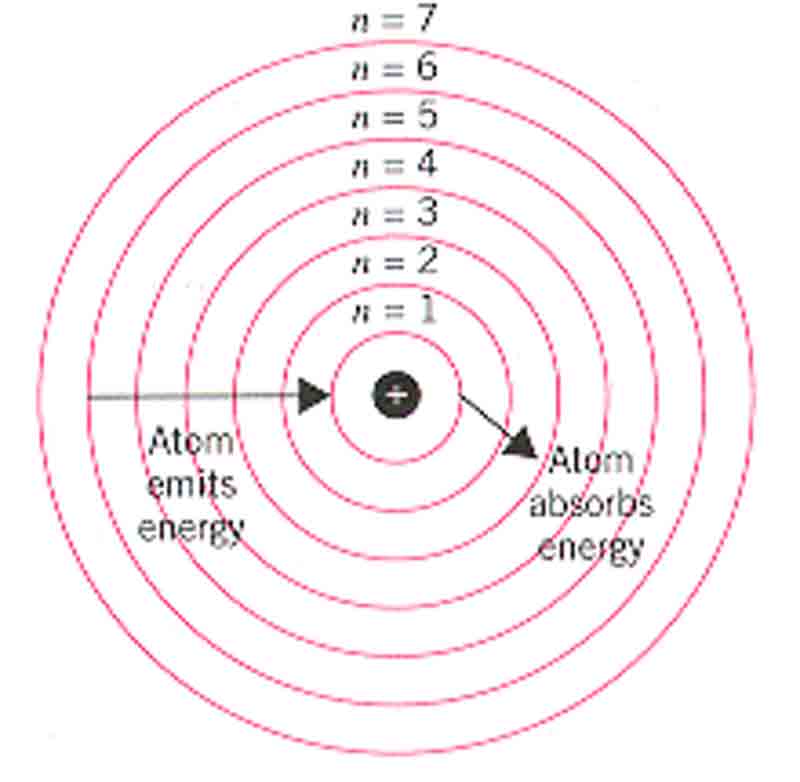
- Electrons level around the nucleus in specific permitted circular orbits and no others. K, L, M, N are respectively defined for n= 1,2,3,4………………………….
- In these particular orbits, an electron does not emit energy. However, an electron can jump to a high energy level by absorbing or falling to lower energy levels by emitting energy.
∆E = E2-E1= hv [ h = Planck’s constant= 6.627×10-34 Js]
- The angular momentum of an electron moving in orbit around the molecules can be expressed as an integral multiple of Planck’s constant divided by 2λ,
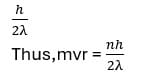
Where m = mass of the electron, r = radius of the electronic orbit, v = velocity of the electron in its orbit.
Limitations of the Bohr’s Model:
- This model is 2D. It does not give any idea for 3D determination.
- This model determines the electron’s particle correctly only. In modern theory, electrons also possess electromagnetic properties.
- The effect of magnetic fields and electric fields splits the spectrum into sharp lines, which is not determined in this model.
- According to the Heisenberg Uncertainty Principle, electrons positioned in an atom at a particular time and momentum cannot be evaluated. Bohr’s model has limits in this section.
The success of Bohr’s model:
- The model can illustrate the spectrum of atoms having one electron (H, He+, L2+ ).
- The radius of the orbit of the electron for the H atom at a lower energy level can be determined which is 0.529 A
- This model indicates the principal quantum number.
- From this model, the revolving electron’s energy in different orbits can get determined or found.
- Ionization potential of H, He+,L2+,B3+ etc. was evaluated from this model.
Failure of Bohr’s Model:
- Bohr’s theory effectively predicted the energy levels for the line spectra of hydrogen, which has a single electron. However, it fell short when explaining the line spectra in atoms with multiple electrons.
- This theory fails to account for the existence of multiple spectral lines.
- This theory fails to account for the splitting of spectral lines observed in magnetic (Zeeman Effect) and electric fields (Stark effect). Additionally, the Bohr atomic model does not adequately explain the intensity of these spectral lines.
- This theory could not explain the dual nature of matter, as described based on the De Broglie concept.
- This theory could not explain the uncertainty principle.
- The concept of energy quantization was left without a definitive conclusion.
Importance of Bohr’s Model:
The Bohr atomic model depicts electrons tracing orbits around the nucleus, similar to how planets revolve around the sun. This model was significant in illustrating that electrons can occupy only certain energy levels. Nevertheless, as additional electrons are incorporated, the model’s accuracy diminishes considerably.