Silicates:
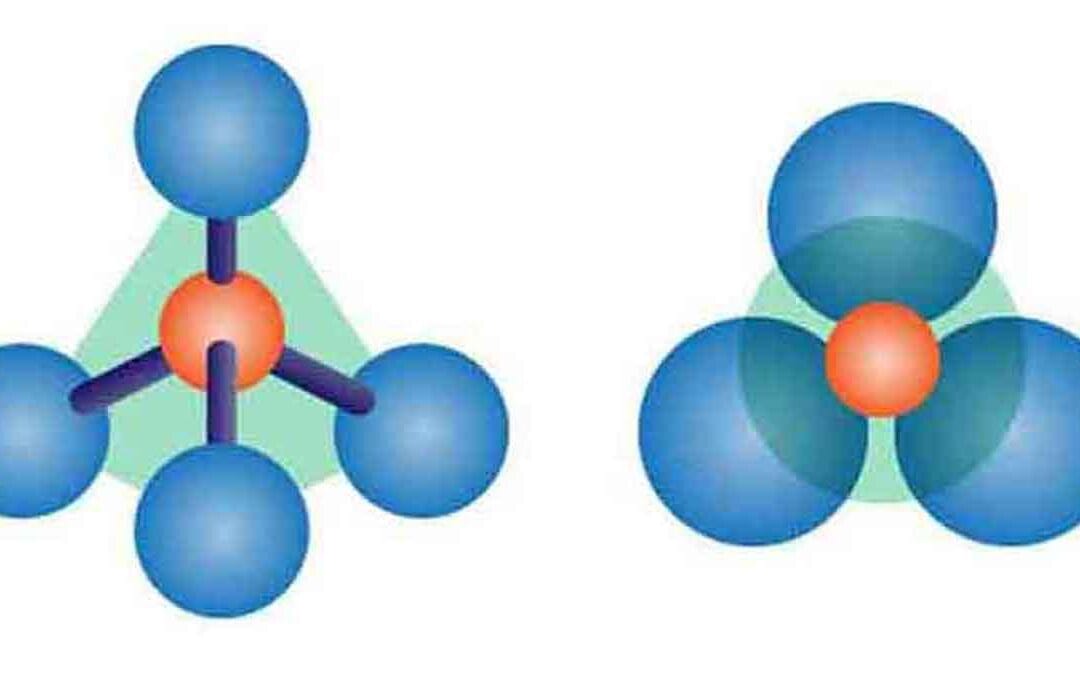
Silicates are compounds containing silicon as an anion. The most common silicates are oxides, but hexafluoro silicates and other anions are also included. Silicate minerals contain (SiO4)4- in their structure.
Silicates form the largest class of mineral species and constitute about 92% of the earth’s crust by volume. The reason behind its abundance in Earth’s crust is:
(i) Oxygen comprises almost half the crust by weight (46.6%), occupying almost 94% by volume, while silicon constitutes about 27% of the crust by weight. Since oxygen and silicon are the most abundant elements, silicate minerals are the most common.
(ii) Silicon combines 4-fold coordination with oxygen, forming silicon-oxygen tetrahedrons linked or polymerized to other cations. Thus, they make various types of silicate compounds.
The silicates are further divided into subclasses based on their structure, i.e., the linkage of silicon-oxygen tetrahedral. The sharing of oxygen may involve one, two, three, or all four oxygen ions in the tetrahedron, giving rise to diverse structural configurations.
The fundamental structural unit of silicates:
Silicate minerals contain (SiO4)4- in their structure. Silicon combines 4-fold coordination with oxygen, forming silicon-oxygen tetrahedron, the fundamental structural unit of silicates. The various ways that (SiO4)4- tetrahedral are linked or polymerized to other cations or other (SiO4)4- tetrahedra.
Classification of silicates:
There are six types of silicates. They are
- Neo silicates or Orthosilicates
- Sorosilicate or Disilicates
- Cyclosilicates
- Inosilicates: (a) Single chained; (b) Double chained
- Phyllosilicates
- Tectosilicates
Neo silicates:
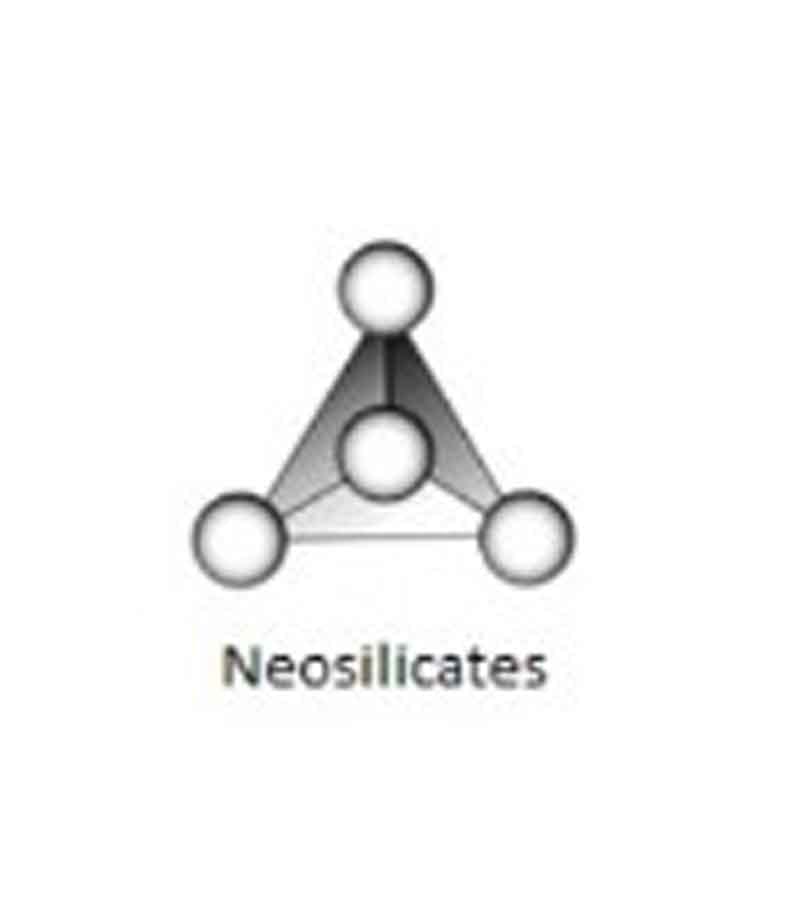
Silicates with independent tetrahedral SiO4 groups in which the tetrahedra are not linked to each other are known as Neo silicates (from the Greek “Nesos”, meaning island) or Orthosilicates (from the Greek “Orthos”, meaning normal)
Examples: Olivine, Forsterite, Fayalite, Garnet, etc.
Si & O ratio: Si: O = 1: 4
Sorosilicate’s:
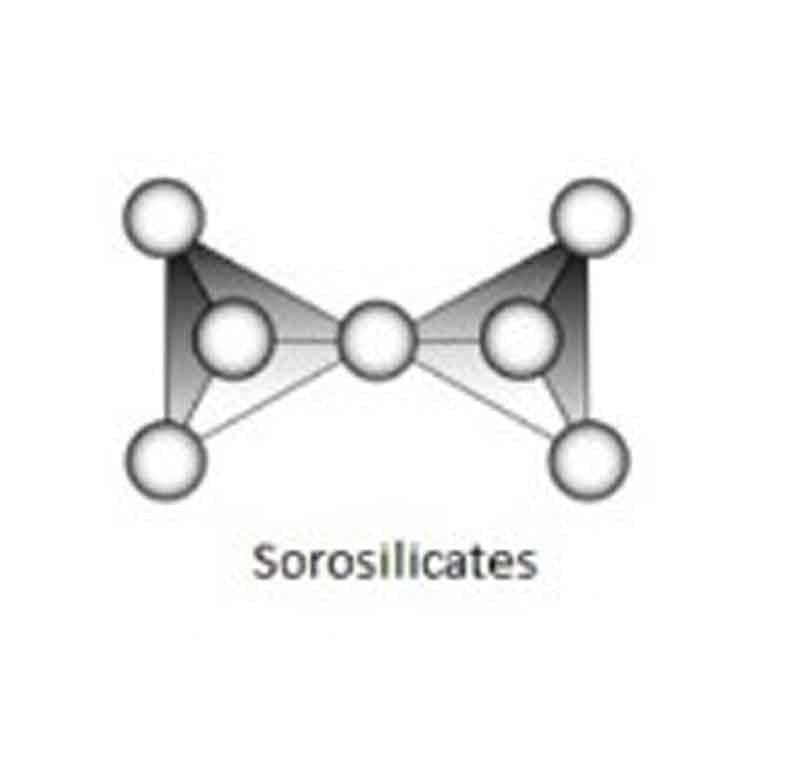
Silicates, in which two SiO4 groups are linked, giving rise to Si2O7 groups, are classed as sorosilicate’s (from the Greek “Soros,” meaning heap) or Disilicates (about the double tetrahedral grouping).
Example: Epidote group, Hemimorphite etc.
Si & O ratio: Si: O = 2: 7
Cyclosilicates:
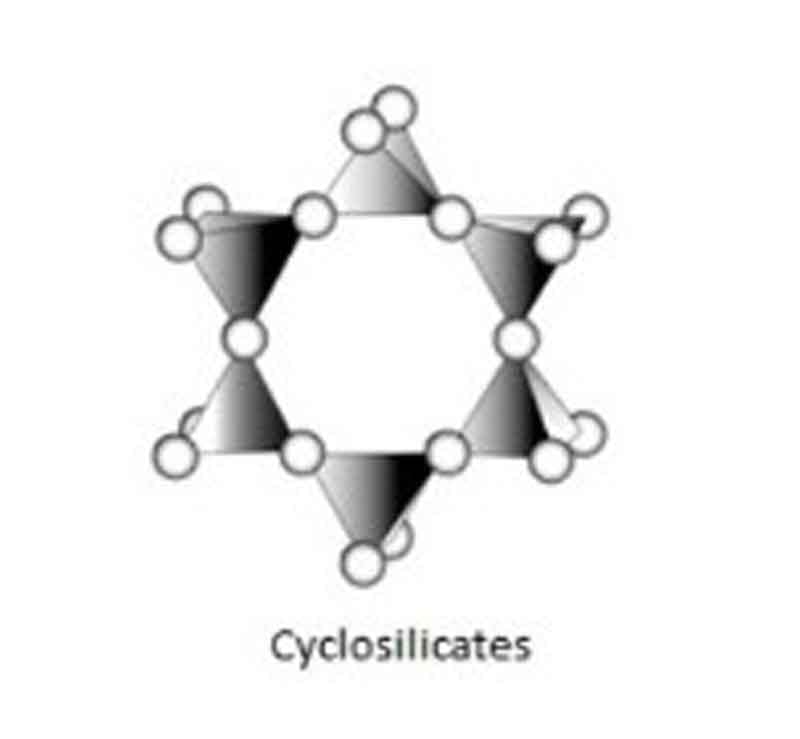
If more than two tetrahedra are linked, closed ring-like structures are formed of a general composition SixO3x. This group is known as the ring silicates, or the Cyclosilicates (from the Greek “Kyklos”, meaning circle)
This group can be divided into three types –
(a) Three-fold tetrahedral ring, which has a composition of Si3O9
(b) Four-fold tetrahedral ring, which has a composition of Si4O12
(c) Six-fold tetrahedral ring, which has a composition of Si6O18
Example: Beryl, Tourmaline, etc.
Si & O ratio: Si: O = 1: 3
Inosilicates:
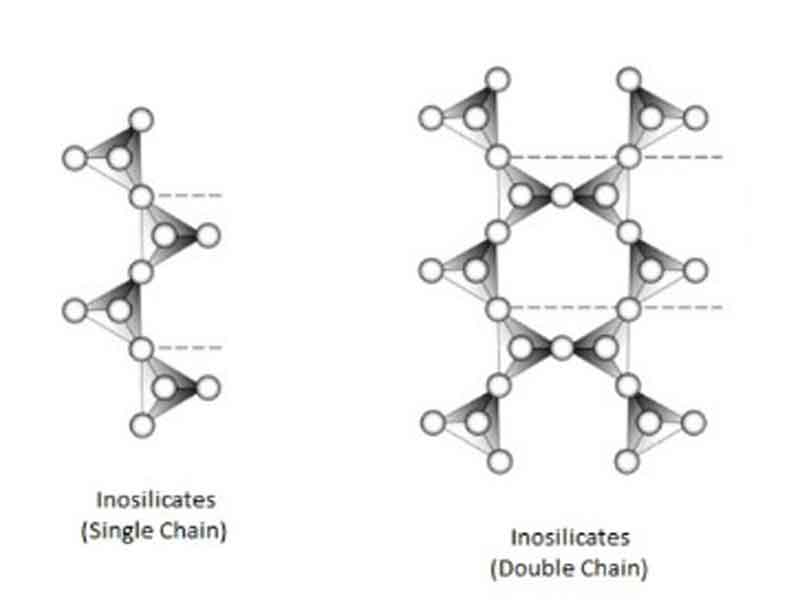
Tetrahedra may also be joined to form infinite single chains with a unit composition of Si2O6 (or SiO3). Infinite double chains give a ratio of Si: O = 4: 11, resulting in Si 4O11 (or Si8O22). Both of these types of chain silicates are also known as inosilicates (from the Greek “Inos,” meaning thread)
This group can be divided into two types –
| (a) Single chain tetrahedra: | Si & O ratio: Si: O = 1: 3 Example: Pyroxene group |
| (b) Double chain tetrahedra: | Si & O ratio: Si: O = 4: 11 Example: Amphibole group |
Phyllosilicates:
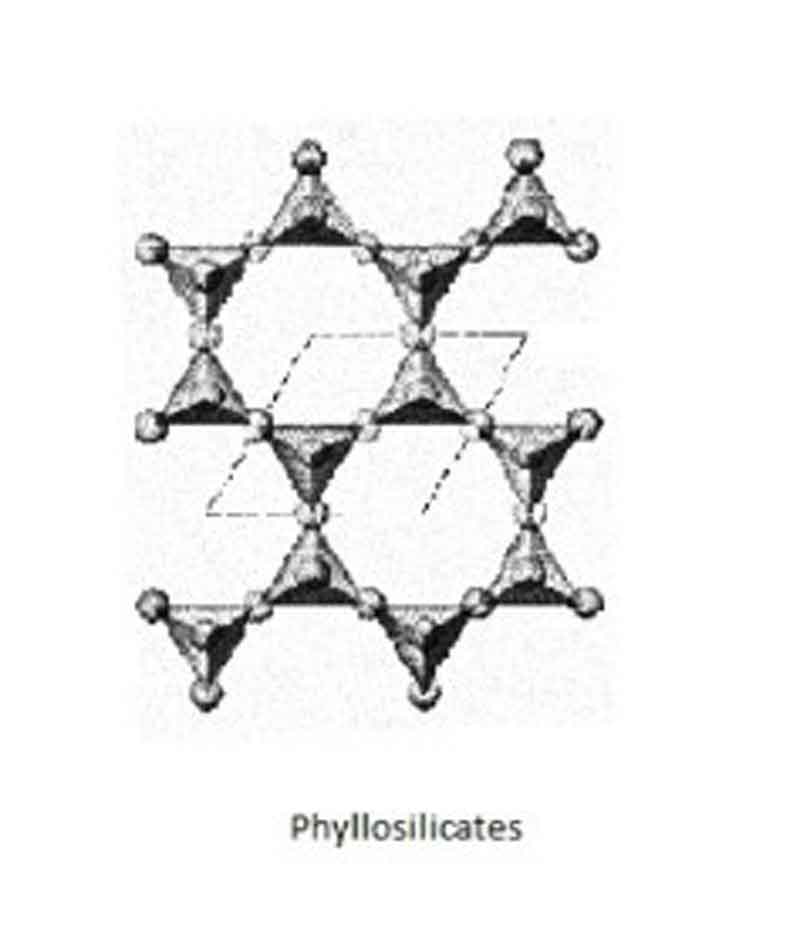
When three of the oxygens of a tetrahedron are shared between adjoining tetrahedra, infinitely extending flat sheets are formed of unit composition Si2O5. Such sheet silicates are also referred to as Phyllosilicates (from the Greek “Phyllon,” meaning leaf)
Examples: Mica group, clay mineral groups, etc.
Si & O ratio: Si: O = 2: 5
Tectosilicates:
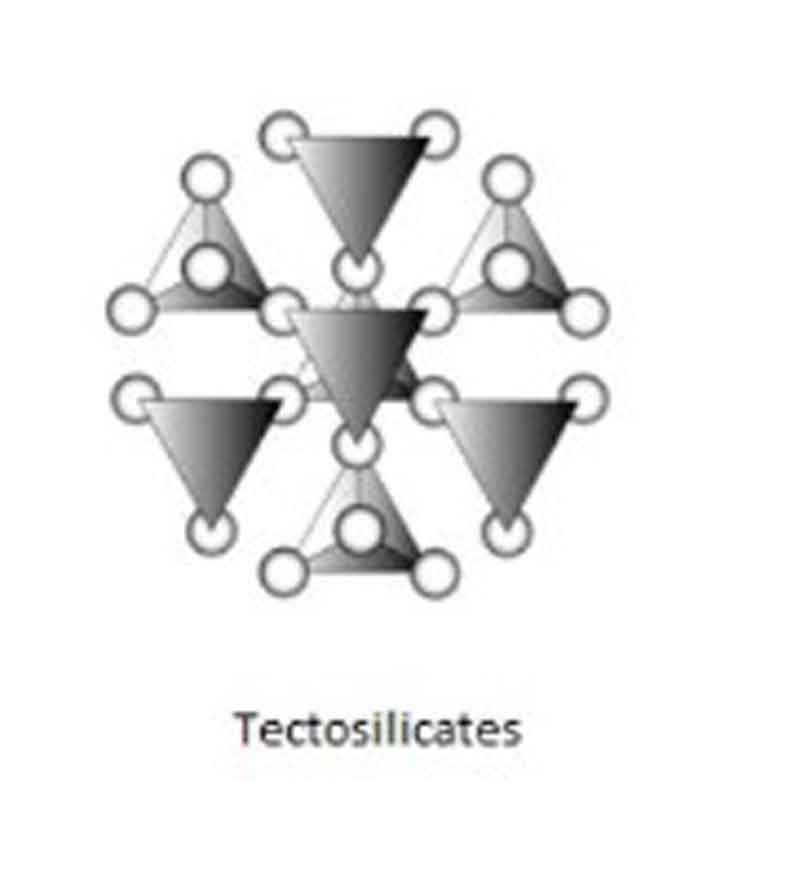
When adjoining tetrahedra, a three-dimensional network of unit composition SiO2 results share all four oxygens of a SiO4 tetrahedron. These framework silicates are known as Tectosilicates (from the Greek “Tecton”, meaning builder)
Examples: Quartz, Feldspar, etc.
Si & O ratio: Si: O = 1: 2