Clay minerals are microscopic, layered silicate minerals that form the fine fraction of soils and sediments. They consist mainly of hydrous aluminum silicates and often include magnesium, iron, and organic materials. Their unique structure made up of tetrahedral and octahedral sheets—controls key properties like plasticity, ion exchange, and water retention. Understanding their composition and structure supports critical applications in soil science, geology, and engineering.
Composition of Clay Minerals:
The word clay refers to a material made up of a mass of small mineral particles. When combined with specific amounts of water, this material shows the property of plasticity. Based on the clay mineral concept, clay materials primarily consist of extremely small crystalline particles from one or more minerals in a small group commonly known as clay minerals.
These minerals typically contain hydrous aluminum silicates, where magnesium or iron may partially or entirely replace aluminum in certain types. Many clay materials also include organic substances and water-soluble salts. Organic matter appears either as discrete particles—like wood, leaf fragments, or spores—or as organic molecules that adhere to the surfaces of clay mineral particles.
Clay may trap water-soluble salts during accumulation, or these salts may form later through groundwater movement and weathering or alteration processes.
Geologists classify clays into three general groups based on their crystalline arrangement. All clay minerals within the same group tend to display similar engineering properties. By initially examining their crystal structure, researchers can better predict how clays will behave under various loading conditions. Fig. 1 lists these groups along with key representative minerals.
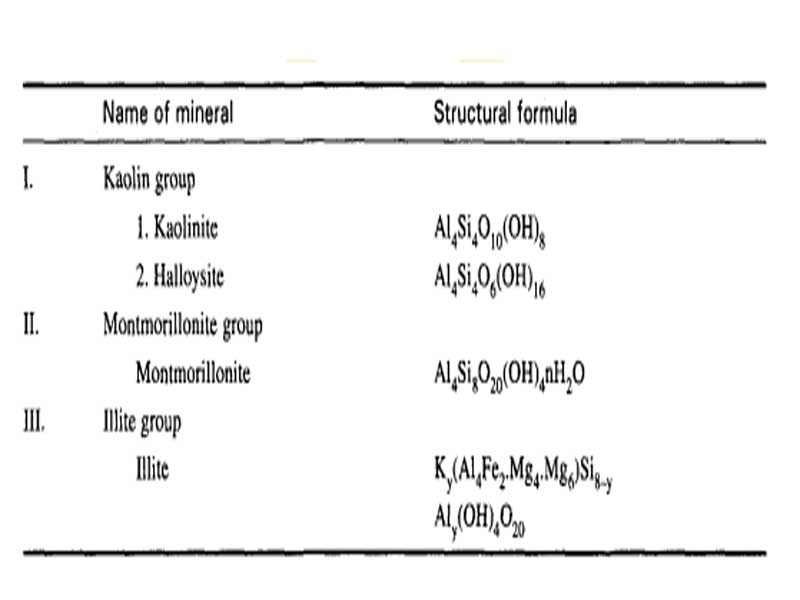
Fig. 1: Clay Minerals
Structure of Clay Minerals:
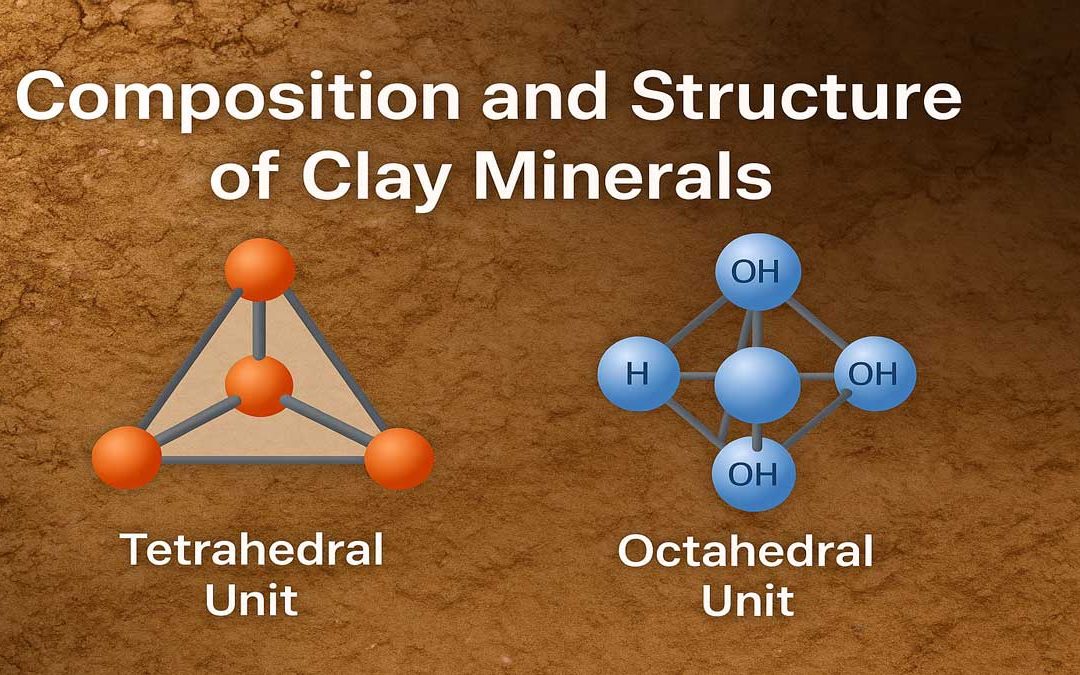
Clay minerals exhibit a crystalline nature, although some, like allophane, also contain non-crystalline material. Two fundamental building blocks form the basis of clay mineral structures:
- Tetrahedral Unit
- Octahedral Unit
Tetrahedral Unit:
This unit includes four oxygen atoms (or hydroxyls, if needed) placed at the corners of a tetrahedron, surrounding a central silicon atom. These tetrahedrons form a shell-like structure with all their tips aligned in the same direction. The oxygen atoms at the base of each tetrahedron lie in a common plane.
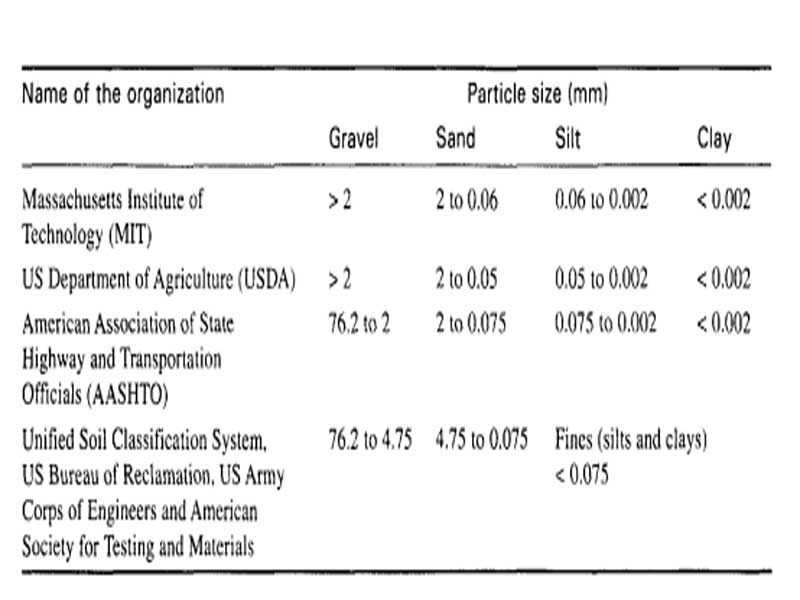
Fig.2: Particle size classification by various systems
Each oxygen ion at the base connects to two tetrahedral units. As shown in Fig. 2 oxygen atoms carry two negative charges, while the silicon atom carries four positive charges. Three oxygen ions at the base share their charges with adjacent units, contributing three negative charges. Along with the two negative charges at the apex, this brings the total to five negative charges per tetrahedral unit. These charges balance the four positive charges from the silicon ion, leaving a net charge of -1 per unit.
Octahedral Unit:
The octahedral unit consists of six hydroxyl ions positioned at the apices of an octahedron, enclosing a central aluminum ion. In some units, magnesium or iron ions replace aluminum. These units form a continuous sheet by sharing hydroxyl ions among three neighboring octahedral units.
This sheet, commonly known as a gibbsite sheet, results in a net charge of +1 per unit when an aluminum ion is at the center. The aluminum ion contributes three positive charges, while each hydroxyl ion shares its single negative charge with two other units—yielding a total of two negative charges per unit [(1/3)×6][(1/3) × 6].
Fig. 1 illustrates this structure. When magnesium replaces aluminum in the octahedral unit, the sheet is called a brucite sheet.
Conclusion:
A clear understanding of the composition and structure of clay minerals helps predict their behavior in both natural and engineered settings. Whether in agriculture, soil science, mineralogy, or civil engineering, clay minerals significantly affect how materials retain water, respond to stress, and perform under different conditions.